Brineura® (cerliponase alfa) is the only treatment that directly addresses the cause of CLN2 disease by helping to replace the TPP1 enzyme
Brineura® (cerliponase alfa) is a type of treatment called enzyme replacement therapy. Brineura helps to replace the TPP1 enzyme, the enzyme that is missing or not working properly in children with CLN2 disease.
Brineura was evaluated in 24 children with CLN2 disease in a clinical study with extension study1
Each child’s ability to walk, with or without assistance, was evaluated over approximately 2 years. Their results were compared to records of untreated patients who experienced the rapid, predictable loss of mobility that occurs in CLN2 disease.1
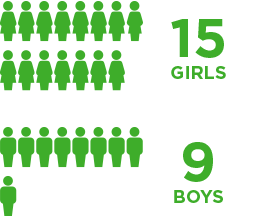
The CLN2 Clinical Rating Scale was used to measure how Brineura works1
Children’s ability to walk and crawl was measured using the CLN2 Clinical Rating Scale. Scores range from 3 (normal) to 0 (loss of walking/crawling abilities).1 Within each score, there may be some differences in the way each child shows their ability to walk or crawl.
Adapted from: Steinfeld R, et al. Am J Med Genet. 2002:112:347-354.
*In some children, walking abilities were never completely normal and were rated as a 2.
Brineura helped maintain children’s ability to walk, with or without assistance, over approximately 2 years of treatment1
In the clinical study, decline was defined as a sustained drop of 2 points or a score of 0 on the CLN2 Clinical Rating Scale.1

- The only Brineura-treated patient who experienced a 2-point decline in their ability to walk or crawl discontinued from the study after 1 infusion1
- Ten children treated with the full dose of Brineura dropped 1 point on the CLN2 Clinical Rating Scale

Layla is a patient with CLN2 disease.
She's been on treatment with Brineura since 2015.
“Layla is touching lives—not only has she touched other people’s lives, I feel like she’s made me a better person. I feel like I’m way more empathetic…Learning and going through this is something that really makes you realize what’s important.”
—Maria, Layla's mom
Possible side effects of Brineura1
Like all medicines, Brineura can cause side effects. Talk to your healthcare team immediately if your child experiences any side effects.
The most common side effects reported during Brineura infusions included1:
- Fever
- Problems with the electrical activity of the heart
- Decreased or increased protein in the fluid of the brain
- Vomiting
- Seizures
- Device-related complications
- Allergic reaction (hypersensitivity)
- Collection of blood outside of blood vessels (hematoma)
- Headache
- Irritability
- Increased white blood cell count in the fluid of the brain
- Device-related infections
- Slow heart rate
- Feeling jittery
- Low blood pressure
If your child is acting differently or if you have any concerns, talk to your healthcare team immediately.
Discover the path toward treatment with Brineura
Every child’s journey with Brineura will be different.
Learn about how to get started.
References: 1. Brineura [package insert]. Novato, CA: BioMarin Pharmaceutical Inc; 2020. 2. Data on file, BioMarin Pharmaceutical Inc. 3. Steinfeld R, Heim P, von Gregory H, et al. Late infantile neuronal ceroid lipofuscinosis: quantitative description of the clinical course in patients with CLN2 mutations. Am J Med Genet. 2002;112:347-354.
