Discover the path toward treatment with Brineura® (cerliponase alfa)
Every child’s journey with Brineura® (cerliponase alfa), an enzyme replacement therapy, will be different. Learn about how to get started.
Your doctor will determine if Brineura is right for your child

DOCTOR’S APPOINTMENT
You'll consult with your child’s doctor and, together, determine whether Brineura is right for your child.
Your child’s doctor may consult with other experts more familiar with treating CLN2 disease. You and your child may need to travel to one of these experts at a different hospital to receive treatment.
Roadmap to Brineura
Your healthcare team at the hospital will work on an individualized plan for your child, which your doctor will discuss with you. This roadmap is meant to give you an overview of the path toward treatment. Your family may have a similar path, or your path may vary.
Your doctor will create a treatment plan specific to your child’s needs
Brineura is a unique therapy, so creating a treatment plan specific to your child’s needs may take some time.
Brineura requires a multidisciplinary team—that means many people from different departments in the hospital will be involved. The hospital will work with your insurance provider to establish reimbursement for Brineura therapy.
As a caregiver, you’re the most important part of your child’s team. You are your child's advocate and a key source of information, whether it’s medical records or insights into your child's well-being.
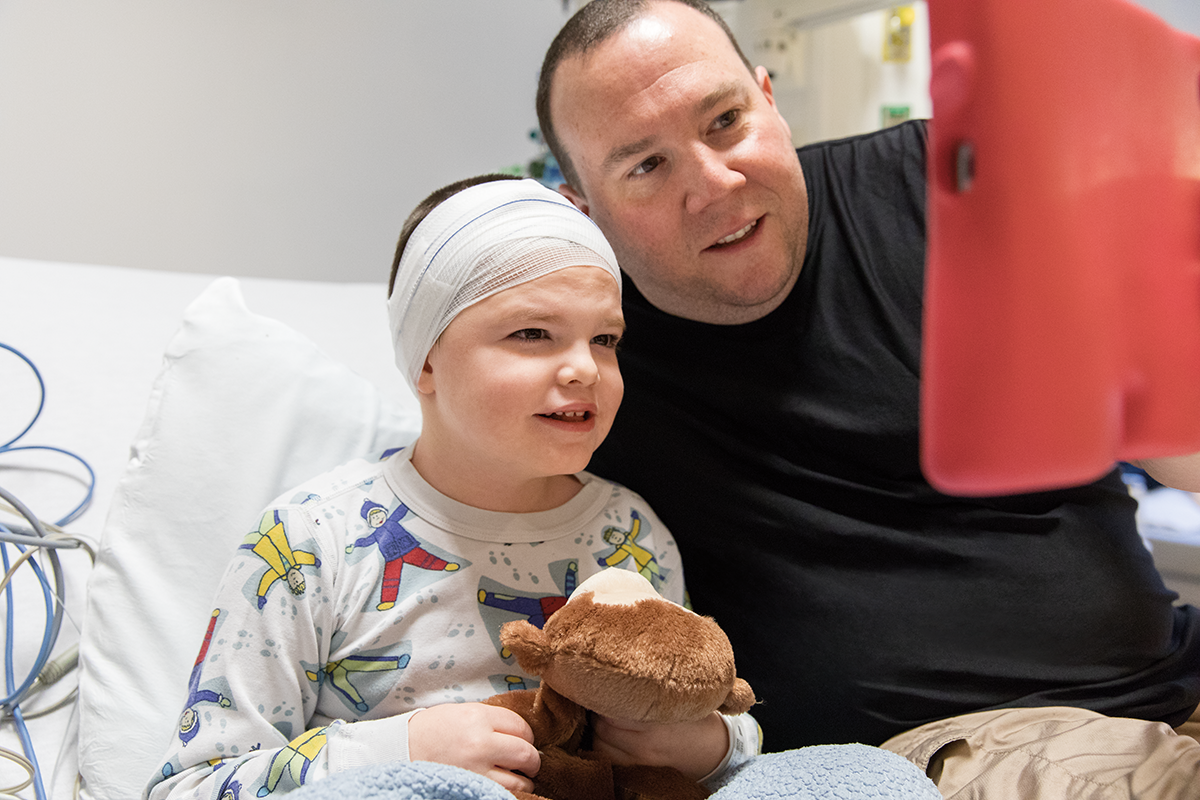
Matty is a patient with CLN2 disease.
He's been on treatment with Brineura since 2015.
On infusion days: “Matty loves his iPad and enjoys playing games, looking at books, and watching movies on it. The iPad makes life so much easier for him (and us) in the hospital.”
Before starting Brineura
Brineura is administered every other week through intraventricular infusion.1 This method allows Brineura to be delivered directly to a ventricle in the brain, and then into the fluid surrounding the brain, known as the cerebrospinal fluid (CSF). Brineura is delivered into the CSF to help reach cells that are affected by CLN2 disease. Knowledgeable members of your healthcare team will give your child’s Brineura infusions1.
To receive intraventricular infusions, your child will first need to have an intraventricular access device surgically implanted.1 This is an established procedure in pediatric neurosurgery.2 The neurosurgeon will discuss the procedure with you and answer any additional questions you may have.
Your healthcare team will let you know how to prepare your child for infusion, and what to expect during this procedure. Your child will be monitored before, during, and after the infusion, and may receive medications to reduce the risk of hypersensitivity reactions.
Low blood pressure and/or slow heart rate, and undesirable hypersensitivity reactions, including fever, vomiting, irritability, and anaphylaxis, may occur during and following the Brineura infusion. Contact your healthcare team immediately if either occur.
After your child has been prescribed Brineura, some of the steps may include:
MRI BRAIN SCANS3
MRI scans are used to help the surgeon locate where the intraventricular access device should be inserted and to confirm placement after surgery.
THE INTRAVENTRICULAR ACCESS DEVICE WILL BE SURGICALLY IMPLANTED
This established procedure is necessary for your child to receive Brineura.1,2 It allows direct delivery of Brineura into a ventricle in the brain.
BRINEURA INFUSIONS CAN BEGIN
It’s recommended that the first dose of Brineura treatment begin at least 5 to 7 days after the access device is implanted.1 Work with your healthcare team to schedule treatments.
Brineura treatment will take about 4.5 hours every other week1
MRI, magnetic resonance imaging.BioMarin RareConnections™ Support Services
Experienced financial navigation support
BioMarin RareConnections provides families with one-to-one financial navigation and logistics support to start and continue therapy.
Learn more about our support services at BioMarin-RareConnections.com.
Connect with BioMarin
Learn more about Brineura by registering to receive updates.
References: 1. Brineura [package insert]. Novato, CA: BioMarin Pharmaceutical Inc; 2020. 2. Cohen-Pfeffer JL, Gururangan S, Lester T, et al. Intracerebroventricular delivery as a safe, long-term route of drug administration. Pediatr Neurol. 2017;67:23-35. 3. Peyrl A, Chocholous M, Azizi AA, et al. Safety of Ommaya reservoirs in children with brain tumors: a 20-year experience with 5472 intraventricular drug administrations in 98 patients. J Neurooncol. 2014;120:139-145.
US-BRIN-00058 0623
